Neuropsychologist Ángel Martínez presents the visuospatial and visuoperceptual alterations present in patients with dementia with Lewy bodies.
Dementia with Lewy bodies
Dementia with Lewy bodies (DLB) is the second most common neurodegenerative dementia after Alzheimer’s Disease, with an estimated prevalence according to different studies between 2% and 25% of all cases (Vann Jones and O’Brien, 2014).
What is dementia with Lewy bodies?
This neurodegenerative disease is mainly characterized by the accumulation of Lewy bodies, composed largely of alpha-synuclein protein, in subcortical regions such as the substantia nigra, the locus coeruleus, the basal nucleus of Meynert and the hypothalamus, as well as in the frontal and temporal cortex, and in the occipital lobes. However, neither the neuropathology, nor the classification, nor the nomenclature of neurodegenerative dementias are straightforward issues; for example, alongside alpha-synuclein deposits there coexist p-Tau and beta-amyloid protein deposits, that is, findings typical of Alzheimer’s disease.
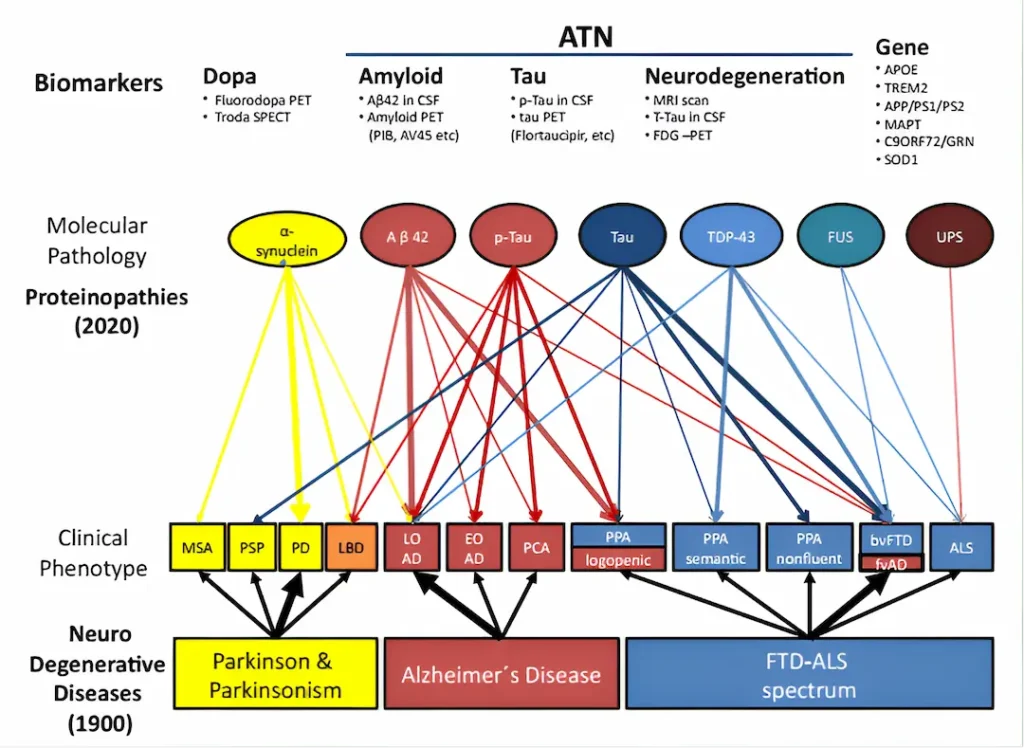
Classification of dementias
In the image, taken from an article with a rather suggestive title, “Moving from neurodegenerative dementias, to cognitive proteinopathies, replacing “where” by “what”” (Allegri, 2020), you can see a classification of neurodegenerative dementias according to the proteins associated with each of them.
Symptoms of dementia with Lewy bodies
Before delving into the description of higher visual processing alterations in patients with dementia with Lewy bodies, and in order to get a general idea of their clinical profile, let’s contextualize them within the set of signs usually reported in the literature as characteristic of this population. Briefly, and according to The Dementia with Lewy Bodies (DLB) Consortium (McKeith et al., 2017), these would be:
- Visual hallucinations.
- Extrapyramidal signs.
- Fluctuation of cognitive state and arousal, apparently similar to delirium.
- Anxiety, depression, delusions and sleep disturbance.
- Prominent cognitive impairment of executive functions, attention, visuoperceptual and visuospatial functions, and, in some publications, visuoconstructive deficits are also included.
Differences between dementia with Lewy bodies and Alzheimer’s Disease
Regarding cognitive aspects, much of the available information about the neuropsychological profile of patients with dementia with Lewy bodies comes from comparative studies or studies aimed at facilitating the early differential diagnosis between this and Alzheimer’s Disease (AD), since they share some similarities in the initial phase.
One of the similarities often mentioned is impairment of episodic memory, although, if we look closely, this similarity is not such, since patients with DLB in mild or prodromal stages show difficulties at the time of information retrieval, improving their performance through the use of cues and recognition of previously presented information, with few intrusions and false positives, versus the predominant deficit in encoding and the commission of numerous intrusions and false positives that characterizes AD (Guidi et al., 2006; Petrova et al., 2016).
In any case, the neuropsychological findings that best define and distinguish dementia with Lewy bodies in its prodromal and mild phases, compared to other dementias and normal aging, are the attentional, executive, visuospatial and visuoperceptual deficits, which stand out relative to the state of other cognitive functions (Gurnani and Gavett, 2017; Kemp et al., 2017). In fact, regarding visuoperceptual deficits, one could say that if amnesia is the hallmark of AD, visual agnosias could be the hallmark of DLB (Ferman et al., 2006; Collerton, et al., 2003).
Focusing on the visuospatial and visuoperceptual deficits, it is estimated that around 70% of patients with dementia with Lewy bodies, compared to 40% of those with Alzheimer’s, present them from the onset of the disease (Wood et al., 2013a).
Visuoperceptual alterations
In the case of visuoperceptual deficits, it has been observed that patients with DLB have difficulties both in simple tasks of discrimination of size, shape and color, that is, difficulties that could be classified as visual pseudo-agnosia according to the classical model of Warrington and Rudge (1995), as well as in complex tasks that involve integration and deeper analysis of visual information, for example, object recognition tasks both in canonical position and in foreshortening, identification of overlapping figures, recognition of objects from a fragment, recognition of fragmented letters, discrimination of real objects from non-objects, or identification of objects from their silhouette (Yokoi et al., 2014), that is, integration apperceptive agnosia and, to a lesser extent, spatial transformation agnosia, according to the model of Humphreys and Riddoch (2013).
In addition, difficulties have also been documented in copying both simple and complex figures, that is, possible visuoconstructive alterations (Kemp et al., 2017). However, in this latter case, a meticulous process-based neuropsychological examination should be considered to refine a differential syndromic diagnosis that clarifies whether difficulties in copying figures are due to constructive apraxia, or, on the contrary, are caused by the visuoperceptual, visuospatial and/or executive difficulties characteristic of patients with dementia with Lewy bodies.
Visuospatial alterations
Regarding visuospatial deficits, difficulties have been described in DLB patients in activities that require identifying the spatial relationship between various visual stimuli, discriminating the angle between lines, performing scanning or visual search, performing a count of the stimuli, motion perception, fitting figures or pieces in 2D, or mentally rotating objects in 3 dimensions.
In reality, there is fairly broad agreement in the description of the visuoperceptual and visuospatial difficulties observed in patients with dementia with Lewy bodies since the vast majority of publications available on this subject in which these signs have been explored or assessed have used the Visual Object and Space Perception Battery (VOSP), overlapping figures tasks and the Hooper Visual Organization Test (Tröster, 2008; Oda et al., 2009; Li et al., 2014, Mitolo et al., 2016). This can be taken as evidence of the very limited variety of neuropsychological tests available for the evaluation of deficits in higher-level visual information processing.

Subscribe
to our
Newsletter
Detection of dementia with Lewy bodies
To the intrinsic value of the early detection, characterization and quantification of cognitive decline in patients with dementia with Lewy bodies we must add a significant added value. Deficits in higher-level visual information processing can play an important role as indicators of patients prognostic evolution in DLB. For example, those patients classified as non-amnestic multidomain mild cognitive impairment that includes visuoperceptual and visuospatial deficits are at higher risk of progressing to dementia with Lewy bodies than those classified as amnestic mild cognitive impairment, who are more likely to progress to Alzheimer’s Disease (Donaghy and McKeith, 2014).
Moreover, those patients who present visuospatial deficits early on are prone to show a more rapid decline both in their ability to perform basic and instrumental activities of daily living, and in their global cognitive status (Hamilton et al., 2008; Wood et al., 2013b).
And, finally, it is important to highlight the relationship between visuoperceptual deficits and visual hallucinations, since the greater the severity of the former as a sign of deterioration of visual association areas, the higher the risk that visual hallucinations will appear later. And this is very important, because visual hallucinations are one of the key signs for the differential diagnosis of DLB versus AD, so early detection of visual agnosias can warn us of what may occur later (Auning et al., 2011).
Neuroanatomical characteristics
Parallel to the study of the neuropsychological profile of patients with dementia with Lewy bodies in the mild or prodromal phase, knowledge has been accumulating about the neuroanatomical characteristics of this disease.
Among its differentiating aspects are signs of early atrophy in the posterior cingulate cortex and in superior temporo-occipital and orbitofrontal regions, along with functional alterations in brain regions necessary for visual information processing, such as the occipital cortex and the occipito-parietal visual association areas (Donaghy and McKeith, 2014; Mak et al., 2014; Yousaf et al., 2019). In addition to the above, we must also include the impairment of cholinergic and dopaminergic pathways due to the action of the Lewy bodies that form in the brainstem. Therefore, these structural and functional alterations would underlie the pattern of functional brain disconnection observed in DLB patients, and would affect the integrity of the ventral occipito-temporal and dorsal occipito-parietal pathways, which are key to supporting visuoperceptual and visuospatial functions (Schumacher et al., 2018).
Conclusion
In the context of a neuropsychological assessment, visuoperceptual and visuospatial alterations are those clinical signs that, if you are able to detect and classify them, will shed a great deal of light on your working hypotheses. In my case, I take them as one who finds a treasure, although I must admit that these clinical signs are especially interesting to me.
On the other hand, I’m not saying anything new if I say that visuoperceptual and visuospatial functions do not top the popularity list of cognitive functions compiled by neuropsychologists, with the consequent problem that what is not attended to is not researched, detected or recognized. Yes, yes, we agree, losing memory is hard, we’re not going to argue that here, but losing the ability to recognize and interpret the world before our eyes, given that we are a species that predominantly explores the world visually, is also no picnic.
Bibliography
- Allegri, R. F. (2020). Moving from neurodegenerative dementias, to cognitive proteinopathies, replacing “where” by “what”…. Dementia & Neuropsychologia, 14(3), 237-242.
- Auning, E., Rongve, A., Fladby, T., Booij, J., Hortobágyi, T., Siepel, F. J., … & Aarsland, D. (2011). Early and presenting symptoms of demen-tia with lewy bodies. Dementia and geriatric cognitive disorders, 32(3), 202-208.
- Collerton, D., Burn, D., McKeith, I., & O’Brien, J. (2003). Systematic review and meta-analysis show that dementia with Lewy bodies is a visual-perceptual and attentional-executive dementia. Dementia and geriatric cognitive disorders, 16(4), 229–237.
- Donaghy, P. C., & McKeith, I. G. (2014). The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimer’s research & therapy, 6(4), 46.
- Ferman, T. J., Smith, G. E., Boeve, B. F., Graff-Radford, N. R., Lucas, J. A., Knopman, D. S., Petersen, R. C., Ivnik, R. J., Wszolek, Z., Uitti, R., & Dickson, D. W. (2006). Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer’s disease. The Clinical neuropsychologist, 20(4), 623–636.
- Guidi, M., Paciaroni, L., Paolini, S., De Padova, S., & Scarpino, O. (2006). Differences and similarities in the neuropsychological profile of dementia with Lewy bodies and Alzheimer’s disease in the early stage. Journal of the Neurological Sciences, 248(1-2), 120-123.
- Gurnani, A. S., & Gavett, B. E. (2017). The differential effects of Alz-heimer’s disease and Lewy Body pathology on cognitive performance: A meta-analysis. Neuropsychology review, 27(1), 1-17.
- Hamilton, J. M., Salmon, D. P., Galasko, D., Raman, R., Emond, J., Hansen, L. A., … & Thal, L. J. (2008). Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology, 22(6), 729.
- Humphreys, G. W., & Riddoch, M. J. (2013). To see but not to see: A case study of visual agnosia. Psychology Press
- Kemp, J., Philippi, N., Phillipps, C., Demuynck, C., Albasser, T., Mar-tin-Hunyadi, C., … & Blanc, F. (2017). Cognitive profile in prodromal dementia with Lewy bodies. Alzheimer’s research & therapy, 9(1), 19.
- McKeith, I. G., Boeve, B. F., Dickson, D. W., Halliday, G., Taylor, J. P., Weintraub, D., … & Kosaka, K. (2017). Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology, 89(1), 88-100.
- Mak, E., Su, L., Williams, G. B., & T O’Brien, J. (2014). Neuroimaging characteristics of dementia with Lewy bodies. Alzheimer’s research & therapy, 6(2), 18.
- Mitolo, M., Hamilton, J. M., Landy, K. M., Hansen, L. A., Galasko, D., Pazzaglia, F., & Salmon, D. P. (2016). Visual perceptual organization ability in autopsy-verified dementia with Lewy bodies and Alzheimer’s disease. Journal of the International Neuropsychological Society, 22(6), 609-619.
- Petrova, M., Pavlova, R., Zhelev, Y., Mehrabian, S., Raycheva, M., & Traykov, L. (2016). Investigation of neuropsychological characteristics of very mild and mild dementia with Lewy bodies. Journal of clinical and experimental neuropsychology, 38(3), 354-360.
- Schumacher, J., Peraza, L. R., Firbank, M., Thomas, A. J., Kaiser, M., Gallagher, P., … & Taylor, J. P. (2018). Functional connectivity in de-mentia with Lewy bodies: A within‐and between‐network analysis. Hu-man brain mapping, 39(3), 1118-1129.
- Tröster, A. I. (2008). Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: differentia-tion, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychology review, 18(1), 103-119.
- Oda, H., Yamamoto, Y., & Maeda, K. (2009). Neuropsychological pro-file of dementia with Lewy bodies. Psychogeriatrics, 9(2), 85-90.
- Vann Jones, S. A., & O’Brien, J. T. (2014). The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychological medicine, 44(4), 673–683.
- Warrington, E. K., & Rudge, P. (1995). A comment on apperceptive agnosia. Brain and cognition, 28(2), 173–179.
- Wood, J. S., Firbank, M. J., Mosimann, U. P., Watson, R., Barber, R., Blamire, A. M., & O’Brien, J. T. (2013a). Testing visual perception in dementia with Lewy bodies and Alzheimer disease. The American Jour-nal of Geriatric Psychiatry, 21(6), 501-508.
- Wood, J. S., Watson, R., Firbank, M. J., Mosimann, U. P., Barber, R., Blamire, A. M., & O’brien, J. T. (2013b). Longitudinal testing of visual perception in dementia with Lewy bodies and Alzheimer’s disease. Inter-national journal of geriatric psychiatry, 28(6), 567-572.
- Yokoi, K., Nishio, Y., Uchiyama, M., Shimomura, T., Iizuka, O., & Mori, E. (2014). Hallucinators find meaning in noises: pareidolic illusions in dementia with Lewy bodies. Neuropsychologia, 56, 245–254.
- Yousaf, T., Dervenoulas, G., Valkimadi, P. E., & Politis, M. (2019). Neuroimaging in Lewy body dementia. Journal of neurology, 266(1), 1–26.
If you liked this post about alterations in patients with dementia with Lewy bodies, you may be interested in these NeuronUP articles.
“This article has been translated. Link to the original article in Spanish:”
Alteraciones en pacientes con demencia por cuerpos de Lewy





 Communication from the caregiver to the patient with dementia
Communication from the caregiver to the patient with dementia
Leave a Reply