PhD candidate Marta Arbizu Gómez presents the recent study “Plasma phospho-tau217 for the diagnosis of Alzheimer’s disease in primary and secondary care using a fully automated platform”, which highlights the accuracy and reliability of the plasma p-tau217 test for diagnosing Alzheimer’s disease.
Why do we need a blood biomarker for Alzheimer’s disease?
Alzheimer’s disease (AD) represents one of the greatest healthcare challenges of the 21st century, as it affects millions of people and places a growing burden on health systems and families.
Traditionally, the definitive diagnosis of AD has relied on invasive techniques (lumbar puncture to analyze cerebrospinal fluid) or costly ones (brain PET scans to visualize amyloid and tau). In addition to being uncomfortable and not always accessible, these procedures often delay diagnostic confirmation until later stages of the disease, when neuronal damage is already significant.
That said, having a reliable, fast, and automatable blood biomarker would make it possible to:
- Detect pathological changes in preclinical or very early stages, opening the door to early interventions.
- Facilitate screening in primary care, easing the load on neurology clinics and reducing waiting times.
- Reduce costs and logistical barriers, by using conventional laboratory platforms without the need for specialized equipment.
The study published on April 9, 2025 in Nature Medicine addresses precisely this need, evaluating for the first time at large scale the potential of plasma phospho-tau217 (p-tau217), measured using a fully automated immunoassay.
How was the research on this plasma p-tau217 test for diagnosing Alzheimer’s carried out?
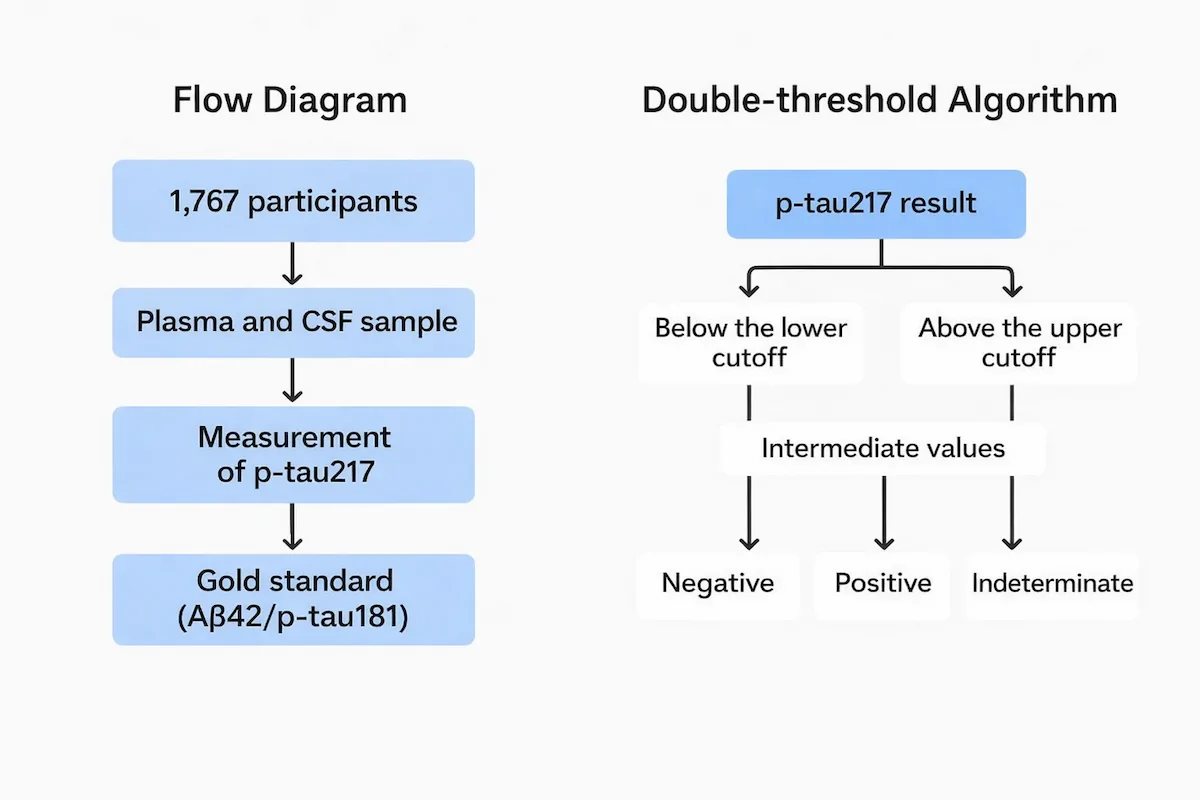
To ensure a rigorous and representative approach, the researchers recruited 1,767 participants with mild cognitive impairment or early dementia from primary and secondary care centers in Sweden, Spain, and Italy. This geographic and care-setting diversity is key to understanding the biomarker’s performance across different clinical environments.
As the “gold standard” to confirm the presence of Alzheimer’s pathology, the study used cerebrospinal fluid analysis with measurement of the classic amyloid-β42 and p-tau181 markers. This allowed each plasma sample to be directly compared with an established diagnosis. Notably, plasma p-tau217 quantification was performed using Fujirebio’s Lumipulse™ platform, a fully automated system that reduces human variability and enables high-throughput processing with strong reproducibility.
This robust design ensures that the results reflect both the immunoassay’s accuracy and its practical applicability in routine laboratories.
What are the key results of the p-tau217 biomarker in diagnosing Alzheimer’s?
The main findings show that the plasma p-tau217 test achieves a diagnostic accuracy of 89–91% in secondary care (neurology and neuropsychology) and 85% in primary care (family physicians). These data confirm that, even without direct access to advanced imaging technology, health centers can reliably identify patients with AD.
Predictive values were also very high: the positive predictive value (PPV) ranged from 82% to 95%, while the negative predictive value (NPV) reached 77% to 90%. In practice, this means that both false positives and false negatives remain low, ensuring effective and safe screening.
A particularly interesting aspect is the biomarker’s robustness, as its performance was not affected by clinical variables such as advanced age (accuracy was 83% in those over 80), the presence of chronic comorbidities, sex, or APOE genotype. In addition, applying a dual-threshold strategy—with a lower and an upper cut-off—raised accuracy to 92–94%, although it left 12% to 17% of samples unclassified (those with intermediate values) to minimize uncertain diagnoses.
| Metric | Value |
|---|---|
| Accuracy in secondary care | 89% – 91% |
| Accuracy in primary care | 85% |
| Positive predictive value (PPV) | 82% – 95% |
| Negative predictive value (NPV) | 77% – 90% |
| Accuracy in participants ≥ 80 years | 83% |
| Accuracy with dual threshold | 92% – 94% |
| Indeterminate samples (intermediate threshold) | 12% – 17% |
What implications does this p-tau217 analysis have for clinical practice?
These results transform the Alzheimer’s diagnostic landscape on multiple fronts:
- Early screening in primary care: the fact that a family physician can request a p-tau217 test and obtain a reliable result prevents unnecessary referrals and speeds up the diagnostic process. This way, the patient reaches specialist care sooner, when intervention is more effective.
- Monitoring and follow-up: a blood biomarker that can be easily repeated makes it possible to assess response to pharmacological treatments or cognitive interventions, as well as monitor disease progression over time, without resorting to invasive tests.
- Standardization and scalability: automation with Lumipulse™ ensures consistent results across laboratories and regions, facilitating multicenter collaboration and the implementation of common protocols within health systems.
Overall, these advantages point to an integrated care model, where biological detection of Alzheimer’s disease is combined with cognitive rehabilitation programs and digital follow-up, offering a holistic approach to the patient.
How does this advance relate to NeuronUP?
NeuronUP develops evidence-based cognitive rehabilitation tools. The integration of biological biomarkers such as p-tau217 complements digital tools by enabling:
- Personalizing programs based on the patient’s actual pathological burden.
- Measuring the impact of interventions not only clinically, but also at a biological level.
- Collaborating with laboratories and medical centers to provide multidisciplinary care that combines early diagnosis and cognitive stimulation.
This study reinforces the vision of comprehensive disease management: while biomedical advances improve detection, NeuronUP enhances functional recovery and quality of life.
Conclusion
The plasma p-tau217 test, validated in this large multicenter study, provides a fast, reliable, and accessible tool for diagnosing Alzheimer’s disease. Its use in primary and secondary care, together with its monitoring potential, opens new pathways for early and personalized care. In the context of NeuronUP, these findings encourage closer collaboration between biological diagnostics and cognitive therapies, advancing toward more comprehensive and effective care.
References
- Palmqvist S, Warmenhoven N, Anastasi F, Pilotto A, Janelidze S, Tideman P, Stomrud E, Mattsson-Carlgren N, Smith R, Ossenkoppele R, Tan K, Dittrich A, Skoog I, Zetterberg H, Quaresima V, Tolassi C, Höglund K, Brugnoni D, Puig-Pijoan A, Fernández-Lebrero A, Contador J, Padovani A, Monane M, Verghese PB, Braunstein JB, Kern S, Blennow K, Ashton NJ, Suárez-Calvet M, Hansson O. Plasma phospho-tau217 for Alzheimer’s disease diagnosis in primary and secondary care using a fully automated platform. Nature Medicine. 2025 Apr 9. doi:10.1038/s41591-025-03622-w.


 Most common errors when starting clinical practice in neuropsychology and how to avoid them
Most common errors when starting clinical practice in neuropsychology and how to avoid them
Leave a Reply